Getting Started
It is never too early to start thinking about quality management since the choices made early in the biodesign innovation process can affect a company’s speed to commercialization and its ability to support large-scale manufacturing. Quality consultant experts are essential in developing an effective quality management system, but they should be engaged once the innovators have a general strategy and approach towards quality management.
Identify Quality Needs and Decide on an Approach
What to Cover
Begin by understanding in what geographic locations the company intends to distribute its products in the next five to seven years. Then, determine which quality system(s) apply (QSR and/or ISO). If the company is at all uncertain about where it will distribute its products, consider building a quality system that is compliant with both. Some elements can always be “turned off” if the alternative system is not required at the time. Understand the requirements of the chosen standard carefully so that an appropriate approach can be decided upon and also in what sequence various components of the quality system need to be fully implemented. Align the stage of the company with the relevant requirements of the quality system so as to establish a strong quality foundation upon which to build as the company grows. Remember that comprehensive risk management strategies are critical to quality systems for companies in all stages of development. There are a wide range of quality publications and software tools designed to help a company understand, implement, and register a quality management system. A range of workshops, seminars, and training courses are also available.
Where to Look
- FDA’s Quality System Regulation Handbook– Provides an overview of good manufacturing practices for manufacturers of medical devices doing business in the U.S.
- FDA’s Quality System Manual – Provides the final rule on 21 CFR Parts 808, 812, and 820, effective October 7, 1996.
- FDA’s Design Control Guidance for Medical Device Manufacturers– Guidance to assist manufacturers in understanding the intent of design control requirements.
- International Organization for Standardization– Provides access to the ISO 13485 standards which set forth quality management requirements for manufacturers of medical devices doing business elsewhere in the world (the standard is for sales on the site). Quality manuals for understanding ISO requirements can also be purchased from many third-party consultants and ISO-accredited certification bodies on the web.
- American Society for Quality – Provides general information about quality, including overview of other quality systems not discussed in this document (e.g., SixSigma). The ASQ store also sells many helpful tools and references.
Hire a Quality Professional
What to Cover
Because so many potential pitfalls exist in implementing a quality system, and because the consequences of noncompliance can be so devastating to a company, it is often a good idea for a company to hire or contract with an experienced quality professional (or team of professionals, depending on the scope of the need) to lead the implementation and ongoing management of a quality system. Companies often work with external quality consultants because they have deep experience and the most current information about effectively navigating quality standards. All management, however, should be trained in quality systems and motivated to lead quality compliance efforts within their areas. Look for a consultant with expertise in the systems the company desires (ISO, QSR, both) and familiarity with the company’s stage of development (start-up, emerging, established). Ask about the consultant’s approach to quality system design. For example, does the person have a “recipe” approach (i.e., one size fits all) or does s/he consider the unique circumstances of the company and the team?
Where to Look
In addition to working with recruiters, reviewing the choices of competitors, and seeking referrals from investors, board members, and other advisors, try making connections through the local chapters of quality associations, such as the ASQ.
Engage Executive and Cross-Functional Management and Define Quality Policies
What to Cover
Responsibility for a quality system begins with executive management. Therefore, it is vital that executive management is involved from the beginning of the process. Meet with executives to ensure their understanding of the management responsibility sections of QSR and/or ISO. Then, initiate the quality system design and implementation process by developing an organizational strategy with top management to engage key players across the company. Assemble a cross-functional team to define the company’s overarching quality policy. The quality policy should set forth the overall intentions and direction of an organization with respect to quality, as established by management with executive responsibility. Validate and reinforce the policy with appropriate employees at all levels within the organization.
Where to Look
Refer to the documentation for the standard being implemented to understand executive management responsibilities. Network with individuals in the field and visit other companies to observe their operations and ask about their approach to quality. Leverage the experience of the company’s quality professional(s).
Build a “Shell” of the Quality System and Develop Elements as the Product Progresses
What to Cover
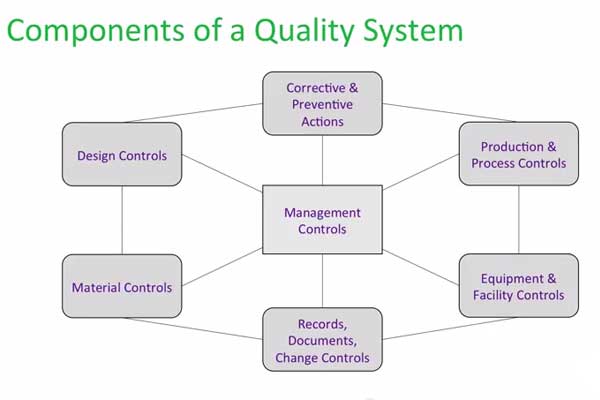
Map out the high-level requirements and processes of the quality system from “end to end” (i.e., from design through manufacturing, distribution, postmarketing surveillance). Then, depending on the stage of the company and its product(s), determine which processes and procedures need to be developed further, at a greater level of detail. Management and design controls are usually the first elements to be developed at the tactical level. Records, documents, and change controls often come next, followed by CAPA processes (to guide continuous improvement), and then P&PC. These elements should be built out (following the guidelines provided in the quality policy and working within the construct of the quality system “shell”) as they are needed, based on the progress of the company.
Where to Look
Leverage the experience of the company’s quality professional(s). Involve the people who will perform the functions where the quality system is being built (e.g., ask R&D engineers to help set up the design control procedures, work with the production manager to design production process and controls). Compliance will be increased if the systems are built by those using them in a way that makes sense in the context of their workflow, and a participative process will yield a better quality system. In addition, a company can leverage a variety of other resources and publications to provide them with relevant information:
- ASQ Biomedical Division Publications
- Textbooks on Quality Systems –
- Marie Teixera and Richard Bradley, Design Controls for the Medical Devices Industry (CRC Press, 2002).
- Michael E. Wiklund, Medical Device and Equipment Design: Usability Engineering and Ergonomics (CRC Press, 1995).
- Basem El-Haik and Khalid S. Mekki, Medical Device Design for Six Sigma: A Road Map for Safety and Effectiveness (John Wiley & Sons, 2008).
- Richard Fries, Reliable Design of Medical Devices (CRC Press, 2006).
Assign Functional Champions to Monitor and Maintain Quality System
What to Cover
While it can be tempting to implement a quality system and then let it run on “auto pilot,” a company must proactively monitor and maintain its quality system to ensure ongoing compliance. Quality systems are meant to be dynamic, changing and improving with the organization as new information, issues, and opportunities are identified. By naming functional champions to oversee the entire quality system, as well as the specific sections most relevant to their individual expertise, the company is more likely to anticipate and prevent challenges and improvements. For this reason, a team approach is typically preferable to assigning a single quality executive to maintain end-to-end oversight of the quality system.
Where to Look
Work with executive managers to identify and empower the appropriate quality champions. The company’s quality professional(s) can help structure these roles, responsibilities, and oversight processes.
Anticipate and Prepare for Audits
What to Cover
Because internal and external audits are an inevitable part of quality management, it is essential to plan for their occurrence and be prepared. Train employees and executives on good audit techniques. This training should be ongoing, not simply performed in advance of a scheduled inspection. Provide representative of the company with the opportunity to practice their audit processes by performing internal inspections on a periodic basis.
Where to Look
Start by reviewing online Appendix 5.5.4. In addition, many external training courses are provided that can be useful in helping employees and professionals prepare for audits (e.g., offered through ASQ). The company’s quality professional(s) can also develop and lead such trainings internally. For more information about FDA’s audit process, refer to FDA’s Quality Systems Manual. ISO training is available through the many ISO-accredited third-party organizations authorized to award ISO certification.